Shock in Pediatrics: Background, Pathophysiology, Etiology
Several etiologic classifications of shock are recognized. The major categories are as follows:
-
Hypovolemic
-
Cardiogenic
-
Distributive
-
Obstructive
In each of these categories of shock, one or more of the physiologic principles that govern oxygen delivery or consumption is disturbed.
Hypovolemia leads to decreased cardiac filling, lower end-diastolic volume, and decreased stroke volume in accordance with the Frank-Starling curve and, therefore, results in decreased cardiac output. Hypovolemia due to hemorrhage additionally decreases oxygen-carrying capacity through the direct loss of available hemoglobin.
Cardiogenic shock resulting from congenital heart disease or cardiomyopathies develops from primary pump failure and inadequate cardiac output.
Distributive shock from sepsis, anaphylaxis, or high-level spinal cord injury results in peripheral vasodilation and decreased systemic vascular resistance (SVR), with venous pooling and inadequate arterial tissue perfusion to meet demand metabolic demands.
Obstructive causes of shock such as pulmonary embolism, pneumothorax, and cardiac tamponade impede either pulmonary outflow, systemic outflow, or both, thereby directly decreasing cardiac output.
Nội Dung Chính
Hypovolemic shock
Hypovolemic shock results from an absolute deficiency of intravascular blood volume. It is a leading cause of pediatric mortality in the United States and worldwide, although the specific causative agents may be different globally. Causes of hypovolemic shock include the following:
-
Intravascular volume loss (eg, from gastroenteritis, burns, diabetes insipidus, heat stroke)
-
Hemorrhage (eg, from trauma, surgery, gastrointestinal bleeding) (see the image below)
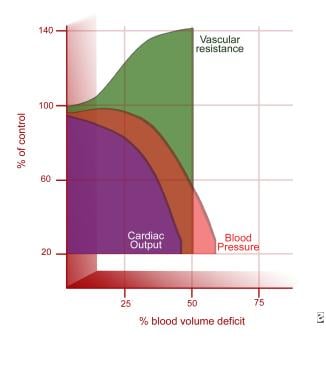
Hemodynamic response to shock hemorrhage model (based on normal data). Adapted from Schwaitzberg SD, Bergman KS, Harris BH. A pediatric trauma model of continuous hemorrhage. J Pediatr Surg. Jul 1988;23(7):605-9.
View Media Gallery
-
Interstitial loss (eg, from burns, sepsis, nephrotic syndrome, intestinal obstruction, ascites)
Gastroenteritis
Children with gastroenteritis may lose 10-20% of their circulating volume within 1-2 hours.
[2] Rehydration is often impeded by concurrent vomiting, and clinical deterioration may be rapid. Common infectious causes of gastroenteritis include bacteria such as Salmonella, Shigella,Campylobacter species (spp), Escherichia coli, and Vibrio cholerae as well as viruses such as rotaviruses, adenoviruses, noroviruses, and enteroviruses. Worldwide, amebiasis and cholera are also important causes.
Hemorrhage
In the United States, the leading cause of death in children younger than 1 year is unintentional injury.
[4] A major component of traumatic death is hemorrhage. In the pediatric patient, primary sites of hemorrhage include intracranial, intrathoracic, intra-abdominal, pelvic, and external. In the pediatric patient in shock without a clear etiology and absent history, occult hemorrhage secondary to nonaccidental trauma should be considered.
[5]
Third spacing
Other causes of hypovolemia include capillary leak and tissue third spacing, which results in leakage of fluid out of the intravascular space into the interstitial tissues. Etiologies include burns, sepsis, and other causes of systemic inflammatory response syndrome (SIRS) (eg, pancreatitis, anaphylaxis). Patients with such etiologies may appear edematous and overloaded with total-body fluid; however, they may be significantly intravascularly depleted, with inadequate preload, and in significant shock. Through understanding of the physiologic disturbance affecting the intravascular volume and preload, it becomes clear that such patients need additional fluid administration despite their overall edematous appearance in order to improve total arterial flow of oxygen (DO2) and to prevent or correct a state of shock.
Distributive shock
In certain clinical states such as distributive shock, normal peripheral vascular tone becomes inappropriately relaxed. Vasodilation results in increased venous capacitance, causing relative hypovolemia even if the patient has not actually had any net fluid loss. As a result, the common physiologic disturbance that affects DO2 in distributive shock is a decrease in preload caused as a result of massive vasodilation and inadequate effective intravascular volume.
Common causes of distributive shock include anaphylaxis, neurologic injury (eg, head injury, spinal shock), sepsis, and drug-related causes.
[6] Causes of anaphylaxis include the following:
-
Medications (eg, antibiotics, vaccines, other drugs)
-
Blood products
-
Envenomation
-
Foods
-
Latex
Anaphylaxis results in mast cell degranulation with resultant histamine release and vasodilation. Neurologic injury can interrupt sympathetic input to vasomotor neurons, resulting in vasodilation. Spinal shock may result from cervical cord injuries above T1, which interrupt the sympathetic chain, allowing for unopposed parasympathetic stimulation. Such patients may present with the clinical picture of hemodynamic instability and hypotension accompanied by bradycardia, because they lose sympathetic vascular tone (resulting in vasodilation) while being unable to mount an appropriate sympathetic-mediated tachycardic response. Medications may also cause vasodilation.
Finally, sepsis causes the release of many vasoactive mediators that may produce profound vasodilation, resulting in distributive shock.
Sepsis
Sepsis may be defined as a dysregulated, systemic inflammatory state that is triggered by the presence of probable or documented infection.
[7, 8] Disturbances of virtually every variable in the DO2 equation may result from the presence of infectious agents such as endotoxin or gram-positive bacterial cell wall components. Systemic molecular cascade activation leads to the release of inflammatory mediators and cytokines (eg, tumor necrosis factor–alpha [TNF-alpha]), interleukins (such as IL-1, IL-2, IL-6), products of the coagulation cascade, bradykinins, and complement activation.
Nitric oxide synthase induction results in production of the potent direct vasodilator nitric oxide, leading to inappropriate and often massive regional and systemic vasodilation. This distributive effect reduces effective preload and impairs cardiac output (CO) and DO2. Circulating toxins and inflammatory mediators can also directly depress myocardial function and reduce cardiac contractility, adding a cardiogenic component to impaired CO. Sepsis may also disrupt capillary integrity, resulting in intravascular fluid leak into tissue third spaces, causing hypovolemia. Overactivation of the clotting cascade can result in disseminated intravascular coagulation (DIC)—DIC can directly obstruct critical tissue capillary beds, resulting in microvascular obstructive shock as well as hemorrhage.
Cardiogenic shock
Impairment of cardiac contractility defines cardiogenic shock. A decreased contractile state results in decreased stroke volume (SV) and CO and, therefore, in decreased DO2. Causes of cardiogenic shock include the following:
-
Arrrhythmias
-
Cardiomyopathies/carditis: Hypoxic/ischemic, infectious, metabolic, connective tissue diseases, neuromuscular disease, toxic reaction, idiopathic
-
Congenital heart disease
-
Trauma
-
Iatrogenic (ie, postoperative low cardiac output syndrome)
Obstructive shock
Obstructive shock occurs when either pulmonary or systemic blood flow is impaired as a result of either congenital or acquired obstruction, leading to CO impairment and shock. Causes include acute cardiac tamponade, tension pneumothorax, massive pulmonary embolism, and other forms of pulmonary or systemic circulation obstruction such as acute or acquired pulmonary hypertension or hypertrophic cardiomyopathy. Additional causes in the neonatal period include coarctation of the aorta, interrupted aortic arch, and severe aortic valvular stenosis.
In addition to medical management for obstructive shock, treatment often depends on prompt recognition and relief of the physical obstruction, such as through pericardiocentesis for tamponade or tube thoracostomy for pneumothorax. Neonates may require maintenance of the patency of the ductus arteriosus in order to bypass the obstruction until more definitive surgery can be performed.